Cleavage of Chordin by Xolloid
Metalloprotease Suggests a Role for Proteolytic Processing in the Regulation of Spemann Organizer Activity
Stefano Piccolo, Eric Agius, Bin Lu, Shelley
Goodman, Leslie Dale, Eddy M. De Robertis
The Xolloid secreted metalloprotease, a -related protein, was found to cleave Chordin and Chordin/BMP-4 complexes at two specific sites in biochemical experiments. Xolloid mRNA blocks secondary axes caused by chordin, but not by noggin, follistatin, or dominant-negative BMP receptor, mRNA injection. Xolloid-treated Chordin protein was unable to antagonize BMP activity. Furthermore, Xolloid digestion released biologically active BMPs from Chordin/BMP inactive complexes. Injection of dominant-negative Xolloid mRNA indicated that the in vivo function of Xolloid is to limit the extent of Spemann’s organizer field. We propose that Xolloid regulates organizer function by a novel proteolytic mechanism involving a double inhibition pathway required to pattern the dorsoventral axis:
![]()
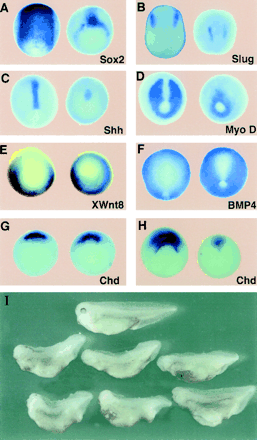 |
Figure
1. Xolloid (XLD) Ventralizes Ectoderm and Mesoderm |
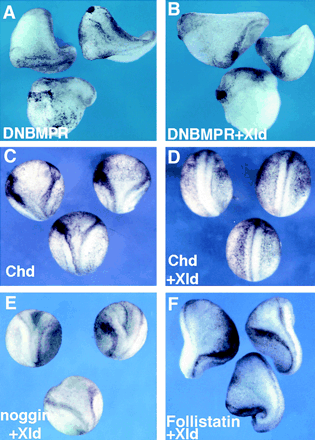 |
Figure 2. Xolloid Acts Upstream of BMP Receptor
and Blocks Secondary Axes Induced by chordin but Not by noggin or follistatin |
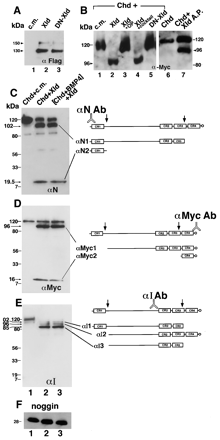 |
Figure
3. Xolloid Is a Secreted Protease That Cleaves Chordin Protein at Two Specific Sites |
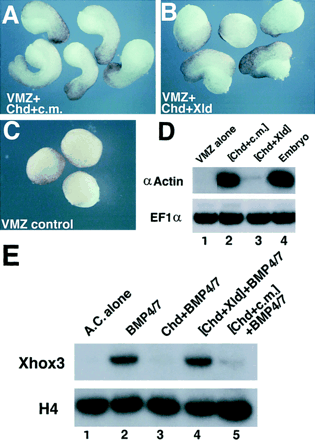 |
Figure
4. Cleavage of Chordin Causes Loss of Dorsalizing and BMP Blocking Activity |
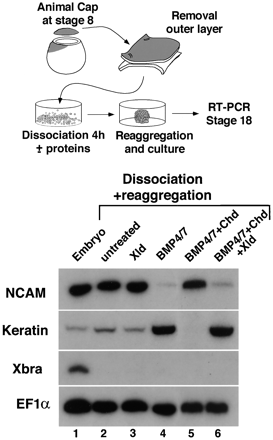 |
Figure 5. Xolloid Digestion Causes the Release
of Biologically Active BMP-4/7 from Inactive CHD/BMP Complexes |
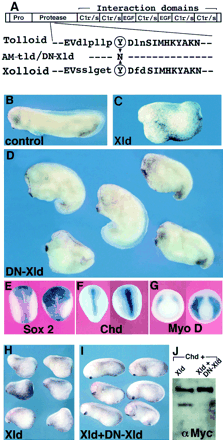 |
Figure
6. In Vivo Role of Xolloid: Expansion of Organizer Activity in Embryos Injected with Dominant-Negative Xolloid |
The establishment of Spemann’s organizer and patterning of the vertebrate embryo.
E. M. De Robertis, Juan Larrain, Michael Oelgeschlager and Oliver Wessely
Nature Reviews Genetics 1, 171-181 (2000)
Molecular studies have begun to unravel the sequential cell-cell signalling events that establish the dorsal-ventral, or ‘back-to-belly’, axis of vertebrate animals. In Xenopus and zebrafish, these events start with the movement of membrane vesicles associated with dorsal determinants. This mediates the induction of mesoderm by generating gradients of growth factors. Dorsal mesoderm then becomes a signalling centre, the Spemann’s organizer, which secretes several antagonists of growth-factor signalling. Recent studies have led to new models for the regulation of cell-cell signalling during development, which may also apply to the homeostasis of adult tissues.
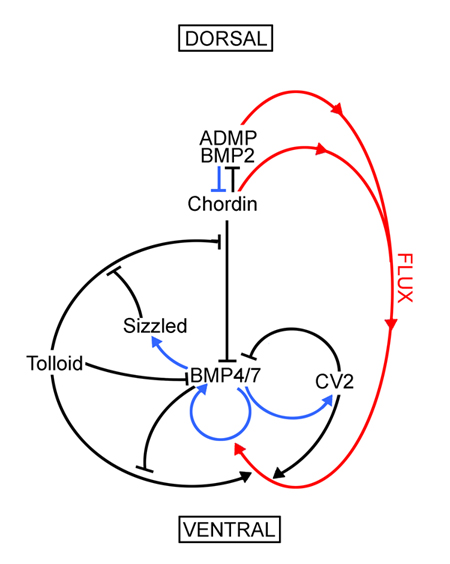
Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field
Bruno Reversade and E. M. De Robertis
Embryos have the ability to self-regulate and regenerate normal structures after being sectioned in half. How is such a morphogenetic field established? We discovered that quadruple knockdown of ADMP and BMP2/4/7 in Xenopus embryos eliminates self-regulation, causing ubiquitous neural induction throughout the ectoderm. ADMP transcription in the Spemann organizer is activated at low BMP levels. When ventral BMP2/4/7 signals are depleted, Admp expression increases, allowing for self-regulation. ADMP has BMP-like activity and signals via the ALK-2 receptor. It is unable to signal dorsally because of inhibition by Chordin. The ventral BMP antagonists Sizzled and Bambi further refine the pattern. By transplanting dorsal or ventral wild-type grafts into ADMP/BMP2/4/7-depleted hosts, we demonstrate that both poles serve as signaling centers that can induce histotypic differentiation over considerable distances. We conclude that dorsal and ventral BMP signals and their extracellular antagonists expressed under opposing transcriptional regulation provide a molecular mechanism for embryonic self-regulation.
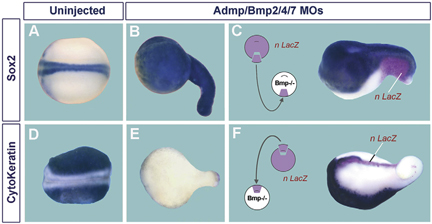 |
Simultaneous depletion of four BMPs causes ubiquitous CNS differentiation, which can be restored by transplantation of either a wild-type ventral center or a dorsal organizer. |
Embryonic dorsal-ventral signaling: secreted Frizzled-related proteins as inhibitors of Tolloid proteinases
Hojoon X. Lee, Andrea L. Ambrosio, Bruno Reversade and E. M. De Robertis
Here we report an unexpected role for the secreted Frizzled-related protein Sizzled/Ogon as an inhibitor of the extracellular proteolytic reaction that controls BMP signaling during Xenopus gastrulation. Microinjection experiments suggest that Sizzled regulates the activity of the Xolloid-related (Xlr) metalloproteinase that degrades Chordin through the following molecular pathway: Szl ┤Xlr ┤Chordin ┤BMP → P-Smad1→ Szl In biochemical assays the Xlr proteinase has similar affinities for itsendogenous substrate Chordin and for its competitive inhibitor Sizzled, which is resistant to degradation by the enzyme. Extracellular levels of Sizzled and Chordin in the gastrula embryo and enzyme reaction constants were all in the 10-8 M range, consistent with a physiological role in the regulation of dorsalventral patterning. Sizzled is also a natural inhibitor of BMP1, a Tolloid metalloproteinase of medical interest. This new layer of regulation mediates intercellular signaling between the dorsal and ventral sides of the embryo.
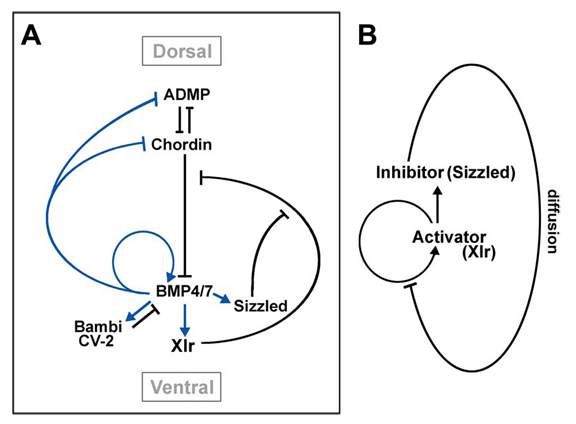 |
Model of the regulation of DV patterning in Xenopus embryos by a network of extracellular proteins.
(A) Digram summarizing the observatins reported here and in Reversade and De Robertis (2005). (B) Digram of how an activator (Xlr) and an inhibitor (Sizzled) would generate pattern, according to the model of Meinhardt and Gierer (2000). |
Enzymatic regulation of pattern: BMP4 binds CUB comains of Tolloids and inhibits proteinase activity
Hojoon X. Lee, Fabio A. Mendes, Jean-Louis Plouhinec and E. M. De Robertis
Genes Dev. 23, 2551-2562 (2009)
In Xenopus embryos, a dorsal-ventral patterning gradient is generated by diffusing Chordin/BMP complexes cleaved by BMP1/Tolloid metalloproteinases in the ventral side. We developed a new BMP1/Tolloid assay using a fluorogenic Chordin peptide substrate and identified an unexpected negative feedback loop for BMP4, in which BMP4 inhibits Tolloid enzyme activity non-competitively. BMP4 binds directly to the CUB domains of BMP1 and Drosophila Tolloid with high affinity. Binding to CUB domains inhibits BMP4 signaling. These findings provide a molecular explanation for a long-standing genetical puzzle in which antimorphic Drosophila mutant alleles displayed anti-BMP effects. The extensive Drosophila genetics available supports the relevance of the interaction described here at endogenous physiological levels. Many extracellular proteins contain CUB domains; the binding of CUB domains to BMP4 suggests a possible general function in binding TGF-β superfamily members. Mathematical modeling indicates that feedback inhibition by BMP ligands acts on the ventral side, while on the dorsal side the main regulator of BMP1/Tolloid enzymatic activity is the binding to its substrate Chordin.
 |
Enzymatic Regulation of Xenopus D-V Patterning. |
