Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann’s organizer
Tewis Bouwmeester, S. Kim, Y. Sasai, B. Lu, and E.M. De Robertis
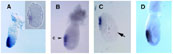
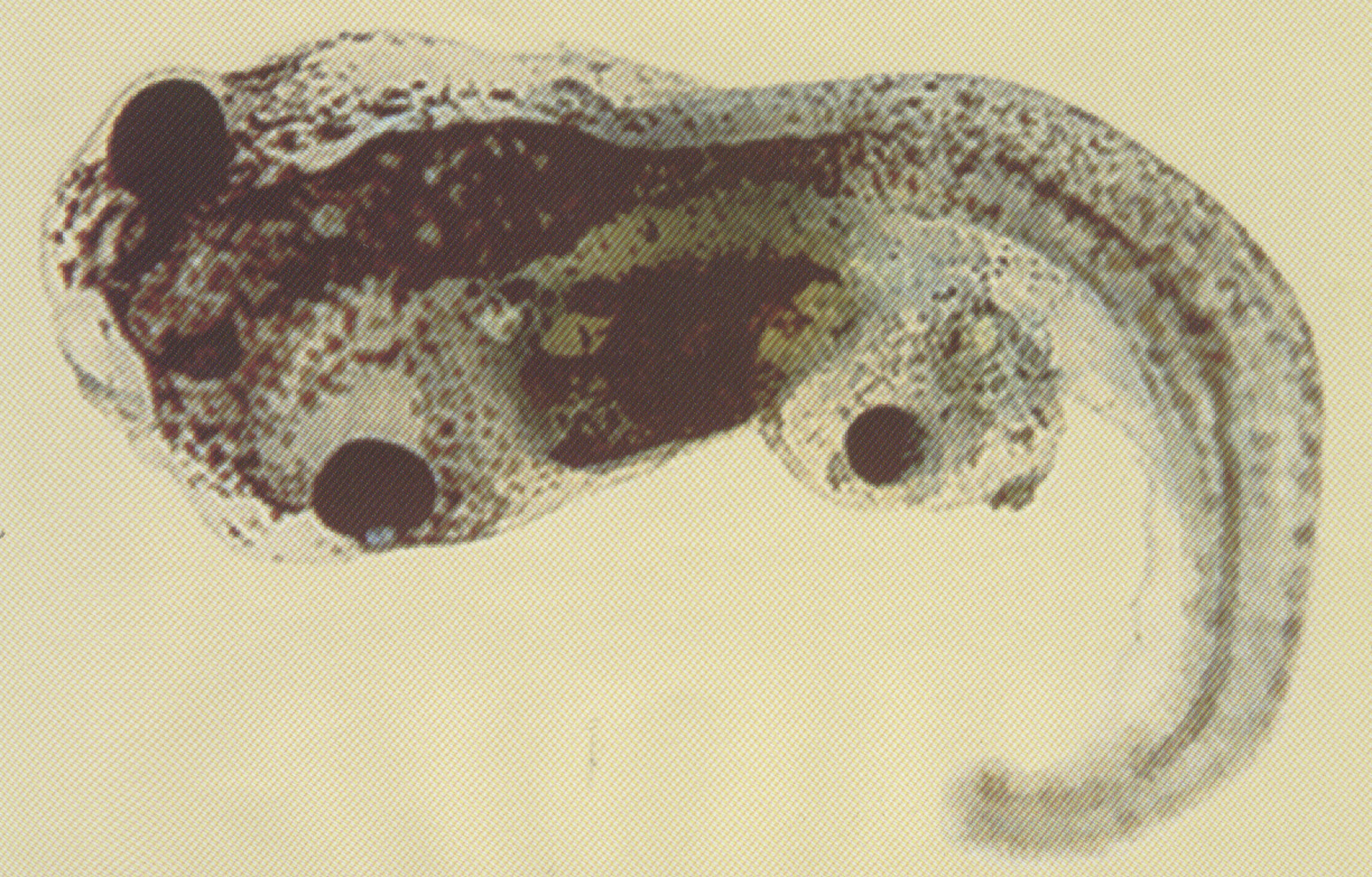
An abundant cDNA enriched in Spemann’s organizer was isolated by differential screening. It encodes a secreted protein that is expressed in the anterior endomesoderm. Microinjection of mRNA into Xenopus embryos induces ectopic heads, and duplicated hearts and livers. The results suggest a role for a molecule expressed in the anterior endoderm in the induction of head structures in the vertebrate embryo.
Cerberus-like is a secreted factor with neuralizing activity expressed in the anterior primitive endoderm of the mouse gastrula
José António Belo, Tewis Bouwmeester, Luc Leyns, Nathalie Kertesz, Michael Gallo, Maximillian Follettie, Eddy M. De Robertis
Mechanisms of Development 68, 45-57 (1997)
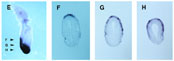
We report the isolation of mouse -like (cer-l), a gene encoding a novel secreted protein that is specifically expressed in the anterior visceral endoderm during early gastrulation. Expression in the primitive endoderm starts before the appearance of the primitive streak and lasts until the head-fold stage. In later stages, a second region of expression is found in newly formed somites. Mouse cer-l shares some sequence similarity with Xenopus (Xcer). In Xenopus assays cer-l, like Xcer, mRNA acts as a potent neuralizing factor that induces forebrain markers and endoderm, but is unable to induce ectopic head-like structures as Xcer does. In addition to cer-l, anterior visceral endoderm was found to express the transcription factors Lim1, goosecoid and HNF-3beta; that are also present in trunk organizer cells. A model of how head and trunk development might be regulated is discussed. Given its neuralizing activity, the secreted protein Cer-l is a candidate for mediating inductive activities of anterior visceral endoderm.
Expression Pattern of mouse Cerberus-like gene during early development.
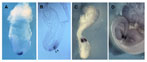
 RT-PCR of frog animal caps injected with mouse Cerberus-like cDNA.
RT-PCR of frog animal caps injected with mouse Cerberus-like cDNA.
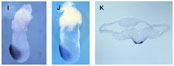
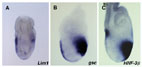 Anterior expression of Lim-1, Goosecoid and HNF3beta.
Anterior expression of Lim-1, Goosecoid and HNF3beta.
The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals
Stefano Piccolo*, Eric Agius*, Luc Leyns,
Subha Bhattacharyya, Horst Grunz, Tewis Bouwmeester & E. M.
De Robertis
* These authors contributed equally to this work
Embryological and genetic evidence indicates that the vertebrate head is induced by a different set of signals from those that organize trunk-tail development. The gene encodes a secreted protein that is expressed in anterior endoderm and has the unique property of inducing ectopic heads in the absence of trunk structures1. Here we show that the protein functions as a multivalent growth-factor antagonist in the extracellular space: it binds to Nodal, BMP and Wnt proteins via independent sites. The expression of during gastrulation is activated by earlier nodal-related signals in endoderm and by Spemann-organizer factors that repress signalling by BMP and Wnt. In order for the head territory to form, we propose that signals involved in trunk development, such as those involving BMP, Wnt and Nodal proteins, must be inhibited in rostral regions.
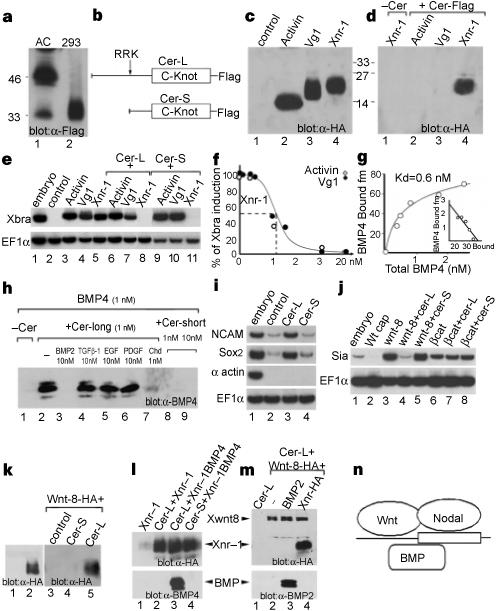 |
Figure 1 Cerberus protein binds to Xnr-1, BMP-4 and Xwnt-8. |
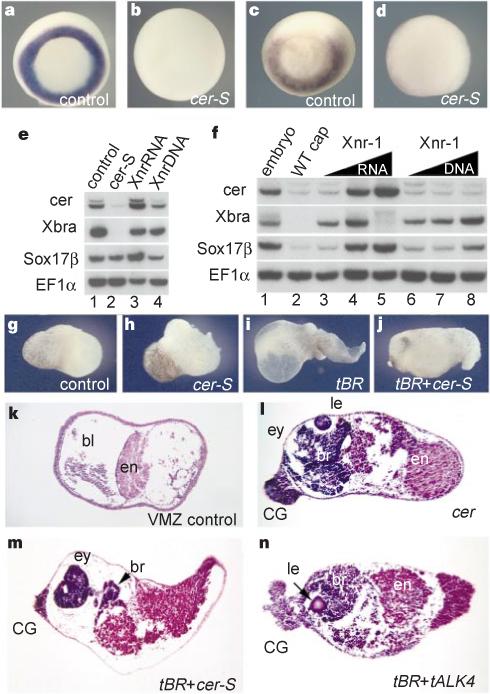 |
Figure 2 Phenotypic effects of cer-S mRNA, an inhibitor of nodal-related signals. |
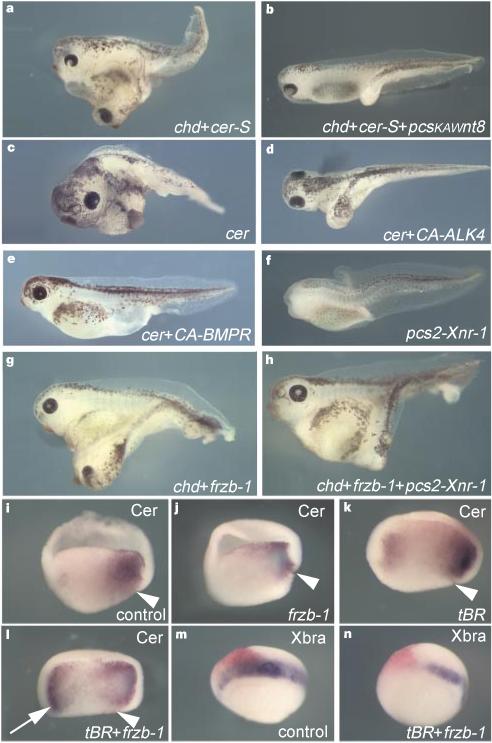 |
Figure 3 Head induction requires the triple inhibition of nodal-related, Wnt and BMP signals. |
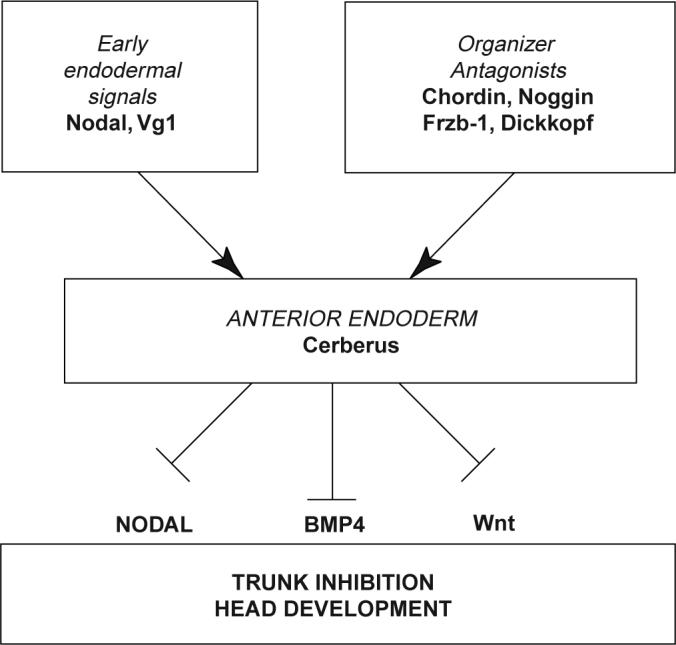 |
Figure 4 Model of the formation and function of anterior endoderm in Xenopus head development
|
.
Cerberus-like is a secreted BMP and nodal antagonist not essential for mouse development
José António Belo, Daniel Bachiller, Eric Agius, Caroline Kemp, A.C. Borges, S. Marques, S. Piccolo S and E.M. De Robertis
Mouse -like (cer-l) is a member of the Cerberus/Dan family of secreted factors. As other members of this family of proteins, Cer-l functions in the extracellular space, inhibiting signaling molecules. Here we show that the neural-inducing and mesoderm-inhibiting activities of Cer-l result from specific binding to BMP and Nodal molecules, respectively. These properties resemble the ones from the related factor Xenopus Cerberus. However, Xenopus Cerberus in addition to BMP4 and Nodal also binds to and inhibits Wnt proteins. We show that Cer-l does not directly inhibit Wnt signals. A null allele of the mouse Cer-l gene was generated by targeted inactivation in ES cells. Homozygous embryos show no anterior patterning defects, are born alive, and are fertile. Since mouse Cer-l and Xenopus Cerberus differ in biochemical activities, we propose the existence of additional members of this family of inhibitors, which may compensate for the loss of cer-l.