Crossveinless-2 is required for the relocalization of Chordin protein within the vertebral field in mouse embryos
Lise Zakin, Ellen Y. Chang, Jean-Louis Plouhinec and E. M. De Robertis
Dev. Biol. 347, 204-215 (2010)
Bone morphogenetic proteins (BMPs), as well as the BMP-binding molecules Chordin (Chd), Crossveinless-2 (CV2) and Twisted Gastrulation (Tsg), are essential for axial skeletal development in the mouse embryo. We previously reported a strong genetic interaction between CV2 and Tsg and proposed a role for this interaction in the shaping of the BMP morphogenetic field during vertebral development. In the present study we investigated the roles of CV2 and Chd in the formation of the vertebral morphogenetic field. We performed immunostainings for CV2 and Chd protein on wild-type, CV2-/- or Chd-/- mouse embryo sections at the stage of onset of the vertebral phenotypes. By comparing mRNA and protein localizations we found that CV2 does not diffuse away from its place of synthesis, the vertebral body. The most interesting finding of this study was that Chd synthesized in the intervertebral disc accumulates in the vertebral body. This relocalization does not take place in CV2-/- mutants. Instead, Chd was found to accumulate at its site of synthesis in CV2-/- embryos. These results indicate a CV2-dependent flow of Chd protein from the intervertebral disc to the vertebral body. Smad1/5/8 phosphorylation was decreased in CV2-/-vertebral bodies. This impaired BMP signaling may result from the decreased levels of Chd/BMP complexes diffusing from the intervertebral region. The data indicate a role for CV2 and Chd in the establishment of the vertebral morphogenetic field through the long-range relocalization of Chd/BMP complexes. The results may have general implications for the formation of embryonic organ-forming morphogenetic fields.
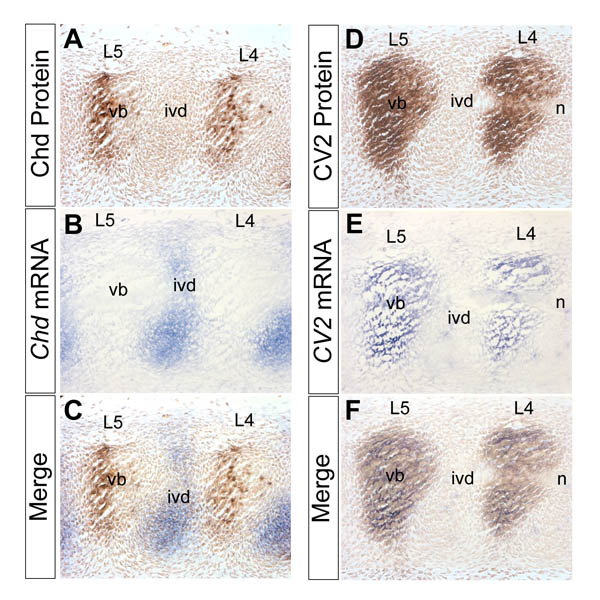
Chd mRNA is synthesized in the prospective intervertebral disc while Chd protein accumulates in the prospective vertebral bodies, in which CV2 mRNA and protein are also found. In situ hybridizations and immunostainings were performed on adjacent saggital sections of a 12.5 d.p.c. wild-type mouse embryo.
Crossveinless-2 is a BMP feedback inhibitor that binds Chordin/BMP to regulate Xenopus embryonic patterning
Andrea L. Ambrosio, Vincent F. Taelman, Hojoon X. Lee, Carrie A. Metzinger, Catherine Coffinier and E. M. De Robertis
Vertebrate Crossveinless-2 (CV2) is a secreted protein that can potentiate or antagonize BMP signaling. Through embryological and biochemical experiments we find that: (1) CV2 functions as a BMP4 feedback inhibitor in ventral regions of the Xenopus embryo; (2) CV2 complexes with Twisted gastrulation and BMP4; (3) CV2 is not a substrate for tolloid proteinases; (4) CV2 binds to purified Chordin protein with high affinity (K(D) in the 1 nM range); (5) CV2 binds even more strongly to Chordin proteolytic fragments resulting from Tolloid digestion or to full-length Chordin/BMP complexes; (6) CV2 depletion causes the Xenopus embryo to become hypersensitive to the anti-BMP effects of Chordin overexpression or tolloid inhibition. We propose that the CV2/Chordin interaction may help coordinate BMP diffusion to the ventral side of the embryo, ensuring that BMPs liberated from Chordin inhibition by tolloid proteolysis cause peak signaling levels.
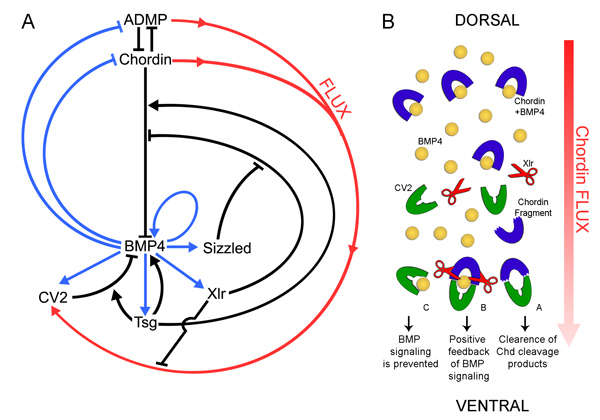
Model of the molecular interaction of CV2, Chordin, Tsg and BMP4
Mouse Crossveinless-2 is the vertebrate homolog of a Drosophila extracellular regulator of BMP signaling
Catherine Coffinier, Nan Ketpura, Uyen Tran, Douglas Geissert and E. M. De Robertis
The Dpp/BMP signaling pathway is highly conserved between vertebrates and invertebrates. The recent molecular characterization of the Drosophila crossveinless-2 (cv-2) mutation by Conley and colleagues introduced a novel regulatory step in the Dpp/BMP pathway (Development 127 (2000) 3945). The CV-2 protein is secreted and contains five cysteine-rich (CR) domains similar to those observed in the BMP antagonist Short gastrulation (Sog) of Drosophila and Chordin (Chd) of vertebrates. The mutant phenotype in Drosophila suggests that CV-2 is required for the differentiation of crossvein structures in the wing which require high Dpp levels. Here we present the mouse and human homologs of the Drosophila cv-2 protein. The mouse gene is located on chromosome 9A3 while the human locus maps on chromosome 7p14. CV-2 is expressed dynamically during mouse development, in particular in regions of high BMP signaling such as the posterior primitive streak, ventral tail bud and prevertebral cartilages. We conclude that CV-2 is an evolutionarily conserved extracellular regulator of the Dpp/BMP signaling pathway.
Development of the vertebral morphogenetic field in the mouse: interactions between Crossveinless-2 and Twisted gastrulation
Lise Zakin, Carrie A. Metzinger, Ellen Y. Chang, Catherine Coffinier and E. M. De Robertis
Crossveinless-2 (Cv2), Twisted Gastrulation (Tsg) and Chordin (Chd) are components of an extracellular biochemical pathway that regulates Bone Morphogenetic Protein (BMP) activity during dorso-ventral patterning of Drosophila and Xenopus embryos, the formation of the fly wing, and mouse skeletogenesis. Because the nature of their genetic interactions remained untested in the mouse, we generated a null allele for Cv2 which was crossed to Tsg and Chd mutants to obtain Cv2; Tsg and Cv2; Chd compound mutants. We found that Cv2 is essential for skeletogenesis as its mutation caused the loss of multiple bone structures and posterior homeotic transformation of the last thoracic vertebra. During early vertebral development, Smad1 phosphorylation in the intervertebral region was decreased in the Cv2 mutant, even though CV2 protein is normally located in the future vertebral bodies. Because Cv2 mutation affects BMP signaling at a distance, this suggested that CV2 is involved in the localization of the BMP morphogenetic signal. Cv2 and Chd mutations did not interact significantly. However, mutation of Tsg was epistatic to all CV2 phenotypes. We propose a model in which CV2 and Tsg participate in the generation of a BMP signaling morphogenetic field during vertebral formation in which CV2 serves to concentrate diffusible Tsg/BMP4 complexes in the vertebral body cartilage.
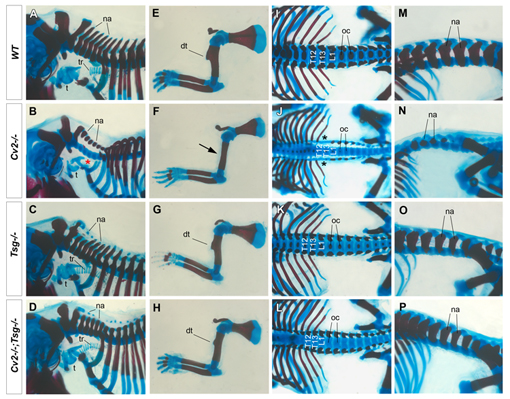
Tsg is epistatic over Cv2: the loss of Tsg in Cv2 -/- suppresses most of the Cv2 mutant skeletal phenotypes.