The role of Paraxial Protocadherin in selective adhesion and cell movements of the mesoderm during Xenopus gastrulation
Sung-Hyun Kim , Akihito Yamamoto , Tewis Bouwmeester
, Eric Agius and E. M. De Robertis
Development 125, 4681-4691 (1998)
Paraxial Protocadherin (PAPC) encodes a transmembrane protein expressed initially in Spemanns organizer and then in paraxial mesoderm. Together with another member of the protocadherin family, Axial Protocadherin (AXPC), it subdivides gastrulating mesoderm into paraxial and axial domains.
PAPC has potent homotypic cell adhesion activity in cell dissociation and reaggregation assays. Gain- and loss-of-function microinjection studies indicate that PAPC plays an important role in the convergence and extension movements that driveXenopus gastrulation. Thus, PAPC is not only an adhesion molecule but also a component of the machinery that drives gastrulation movements in Xenopus. PAPC may provide a link between regulatory genes in Spemanns organizer and the execution of cell behaviors during morphogenesis.
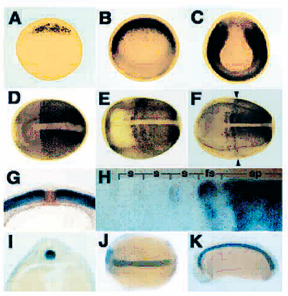 |
Fig. 2. PAPC is expressed during mesodermal mantle morphogenesis. |
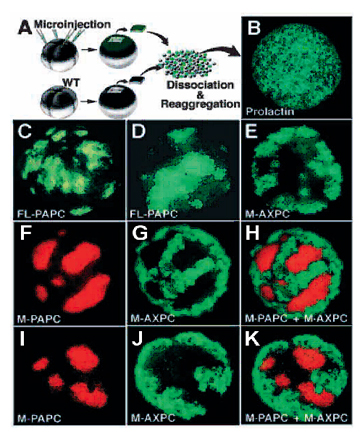 |
Fig. 3. PAPC causes homotypic cell sorting in reaggregation experiments. |
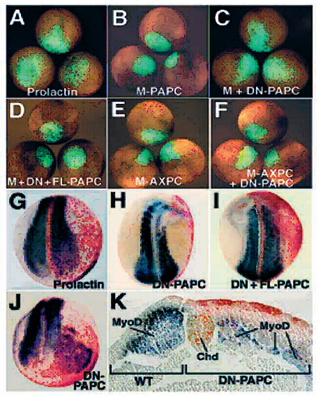 |
Fig. 4. Dominant-negative PAPC affects paraxial mesoderm morphogenesis. |
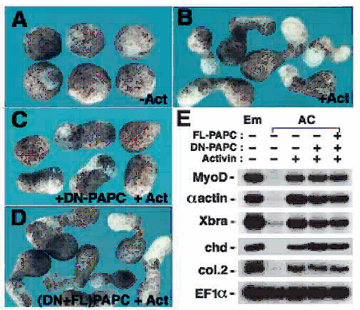 |
Fig. 5. DN-PAPC mRNA inhibits animal cap extension by activin. |
 |
Fig. 6. FL-PAPC can promote animal cap elongation and changes in cell morphology. |
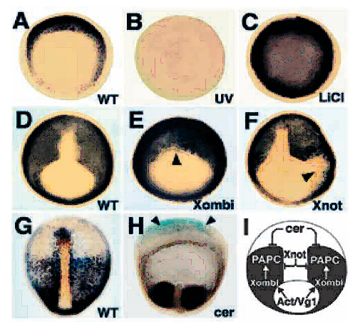 |
Fig. 7. Multiple regulatory steps control PAPC expression. |
Zebrafish paraxial protocadherin is a downstream target of spadetail involved in morphogenesis of gastrula mesoderm
Akihito Yamamoto, Sharon L. Amacher, Sung-Hyun Kim, Douglas
Geissert, Charles B. Kimmel and E. M. De Robertis
Development 125, 3389-3397 (1998)
Zebrafish paraxial protocadherin () encodes a transmembrane cell adhesion molecule (PAPC) expressed in trunk mesoderm undergoing morphogenesis. Microinjection studies with a dominant-negative secreted construct suggest that is required for proper dorsal convergence movements during gastrulation. Genetic studies show that is a close downstream target of spadetail, gene encoding a transcription factor required for mesodermal morphogenetic movements. Further, we show that the floating head homeobox gene is required in axial mesoderm to repress the expression of both spadetail and , promoting notochord and blocking differentiation of paraxial mesoderm. The PAPC structural cell-surface protein may provide a link between regulatory transcription factors and the actual cell biological behaviors that execute morphogenesis during gastrulation.
The protocadherin PAPC establishes segmental boundaries during somitogenesis in xenopus embryos
Sung H. Kim, W.C. Jen, E.M. De Robertis and Chris Kintner
Current Biology 10, 821-830 (2000)
One prominent example of segmentation in vertebrate embryos is the subdivision of the paraxial mesoderm into repeating, metameric structures called somites. During this process, cells in the presomitic mesoderm (PSM) are first patterned into segments leading secondarily to differences required for somite morphogenesis such as the formation of segmental boundaries. Recent studies have shown that a segmental pattern is generated in the PSM of Xenopus embryos by genes encoding a Mesp-like bHLH protein called Thylacine 1 and components of the Notch signaling pathway. These genes establish a repeating pattern of gene expression that subdivides cells in the PSM into anterior and posterior half segments, but how this pattern of gene expression leads to segmental boundaries is unknown. Recently, a member of the protocadherin family of cell adhesion molecules, called PAPC, has been shown to be expressed in the PSM of Xenopus embryos in a half segment pattern, suggesting that it could play a role in restricting cell mixing at the anterior segmental boundary. RESULTS: Here, we examine the expression and function of PAPC during segmentation of the paraxial mesoderm in Xenopus embryos. We show that Thylacine 1 and the Notch pathway establish segment identity one segment prior to the segmental expression of PAPC. Altering segmental identity in embryos by perturbing the activity of Thylacine 1 and the Notch pathway, or by treatment with a protein synthesis inhibitor, cycloheximide, leads to the predicted changes in the segmental expression of PAPC. By disrupting PAPC function in embryos using a putative dominant-negative or an activated form of PAPC, we show that segmental PAPC activity is required for proper somite formation as well as for maintaining segmental gene expression within the PSM.
CONCLUSIONS: Segmental expression of PAPC is established in the PSM as a downstream consequence of segmental patterning by Thylacine 1 and the Notch pathway. We propose that PAPC is part of the mechanism that establishes the segmental boundaries between posterior and anterior cells in adjacent segments.
Mouse paraxial protocadherin is expressed in trunk mesoderm and is not essential for mouse development
Akihito Yamamoto, Caroline Kemp, Daniel Bachiller, Douglas Geissert and E.M. De Robertis
Paraxial protocadherin (PAPC) is a cell adhesion molecule that marks cells undergoing convergence-extension cell movements in Xenopus and zebrafish gastrulating embryos. Here a mouse homologue (m) was identified and characterized. During early- to mid-gastrulation, m is expressed in the primitive streak as the trunk mesoderm undergoes morphogenetic cell movements. At head-fold stage m expression becomes localized to paraxial regions in which somites are formed in the segmental plate. At later stages, m displays a complex expression pattern in cerebral cortex, olfactory bulb, inferior colliculus, and in longitudinal stripes in hindbrain. To analyze the effect of the loss of PAPC function during mouse development, a null allele of the mouse gene was generated. Homozygous animals show no defects in their skeleton and are viable and fertile.
Analysis of Spemann Organizer Formation in Xenopus Embryos by cDNA Macroarrays
Oliver Wessely, James I. Kim, Douglas Geissert, Uyen
Tran, and E. M. De Robertis
Developmental Biology 269, 552-566 (2004)
The understanding of vertebrate development has greatly benefited from the study of gastrulation in the Xenopus embryo. Over the years the molecular dissection of the Spemann organizer has proven to be a very fruitful source for gene discovery. Here we report a comprehensive screen of gene expression in the Xenopus gastrula using cDNA macroarrays. Nylon filters containing more than 72,000 cDNAs from a gastrula stage library were hybridized with differential probes from embryos in which organizer induction had been inhibited by reducing Nodal-related or maternal -Catenin signaling. Combining the changes in gene expression levels caused by these two major signaling pathways in a single graph identified both known and novel dorso-ventral regulated genes. The most highly enriched organizer-specific genes were the secreted molecules chordin and Xnr-3, followed by the transmembrane protein paraxial protocadherin (PAPC). Ventral-specific abundant cDNAs included S10-40-H5, members of the Hyaluronan synthase family, Xvent-2 and Xfd2/FoxI1. A differential probe of dorsal and ventral lips identified many more organizer-specific cDNAs than the screens inhibiting Nodal-related and -Catenin signaling, suggesting that additional, as yet uncharacterized signaling pathways, contribute to organizer formation. Finally, extension of this approach to the blastula preorganizer signaling center identified the transcription factor pintallavis/FoxA2 as a new preorganizer component.
Paraxial protocadherin coordinates cell polarity during convergent extension via Rho A and JNK
F. Unterseher, J.A. Hefele, K. Giehl, E.M. De Robertis, Doris Wedlich and A. Schambony
EMBO Journal 23, 3259-3269 (2004)
Convergent extension movements occur ubiquitously in animal development. This special type of cell movement is controlled by the Wnt/planar cell polarity (PCP) pathway. Here we show that Xenopus paraxial protocadherin (XPAPC) functionally interacts with the Wnt/PCP pathway in the control of convergence and extension (CE) movements in Xenopus laevis. XPAPC functions as a signalling molecule that coordinates cell polarity of the involuting mesoderm in mediolateral orientation and thus selectively promotes convergence in CE movements. XPAPC signals through the small GTPases Rho A and Rac 1 and c-jun N-terminal kinase (JNK). Loss of XPAPC function blocks Rho A-mediated JNK activation. Despite common downstream components, XPAPC and Wnt/PCP signalling are not redundant, and the activity of both, XPAPC and PCP signalling, is required to coordinate CE movements.
Activity-induced protocadherin Arcadlin regulates dendritic spine number by triggering N-Cadherin endocytosis via TAO2b and p38 MAP kinases
S. Yasuda, H. Tanaka, H. Sugiura, K. Okamura, T. Sakaguchi, Uyen Tran, T. Takemiya, A. Mizoguchi, Y. Yagita, T. Sakurai, E.M. De Robertis and K. Yamagata
Synaptic activity induces changes in the number of dendritic spines. Here, we report a pathway of regulated endocytosis triggered by arcadlin, a protocadherin induced by electroconvulsive and other excitatory stimuli in hippocampal neurons. The homophilic binding of extracellular arcadlin domains activates TAO2beta, a splice variant of the thousand and one amino acid protein kinase 2, cloned here by virtue of its binding to the arcadlin intracellular domain. TAO2beta is a MAPKKK that activates the MEK3 MAPKK, which phosphorylates the p38 MAPK. Activation of p38 feeds-back on TAO2beta, phosphorylating a key serine required for triggering endocytosis of N-cadherin at the synapse. Arcadlin knockout increases the number of dendritic spines, and the phenotype is rescued by siRNA knockdown of N-cadherin. This pathway of regulated endocytosis of N-cadherin via protocadherin/TAO2beta/MEK3/p38 provides a molecular mechanism for transducing neuronal activity into changes in synaptic morphologies.