Dorsal-Ventral patterning: is a dorsally secreted Frizzled-related protein that competitively inhibits Tolloid proteases
Diego Ploper, Hojoon X. Lee and Edward M. De Robertis
Dev. Biol. 352, 317-328 (2011)
In Xenopus, dorsal-ventral (D-V) patterning can self-regulate after embryo bisection. This is mediated by an extracellular network of proteins secreted by the dorsal and ventral centers of the gastrula. Different proteins of similar activity can be secreted at these two poles, but under opposite transcriptional control. Here we show that , a dorsal protein, can compensate for the loss of Sizzled, a ventral protein. is a secreted Frizzled-Related Protein (sFRP) known to regulate Wnt8 and Wnt11 activity. We now find that also regulates the BMP pathway. expression was increased by the BMP antagonist Chordin and repressed by BMP4, while the opposite was true for Sizzled. knock-down increased the expression of BMP target genes, and synergized with Sizzled morpholinos. Thus, loss-of-function is compensated by increased expression of its ventral counterpart Sizzled. overexpression dorsalized whole embryos but not ventral half-embryos, indicating that requires a dorsal component to exert its anti-BMP activity. protein lost its dorsalizing activity in Chordin-depleted embryos. When co-injected, and Chordin proteins greatly synergized in the dorsalization of Xenopus embryos. The molecular mechanism of these phenotypes is explained by the ability of to inhibit Tolloid metalloproteinases, which normally degrade Chordin. Enzyme kinetic studies showed that was a competitive inhibitor of Tolloid activity, which bound to Tolloid/BMP1 with a KD of 11 nM. In sum, is a new component of the D-V pathway, which functions as the dorsal counterpart of Sizzled, through the regulation of chordinases of the Tolloid family.
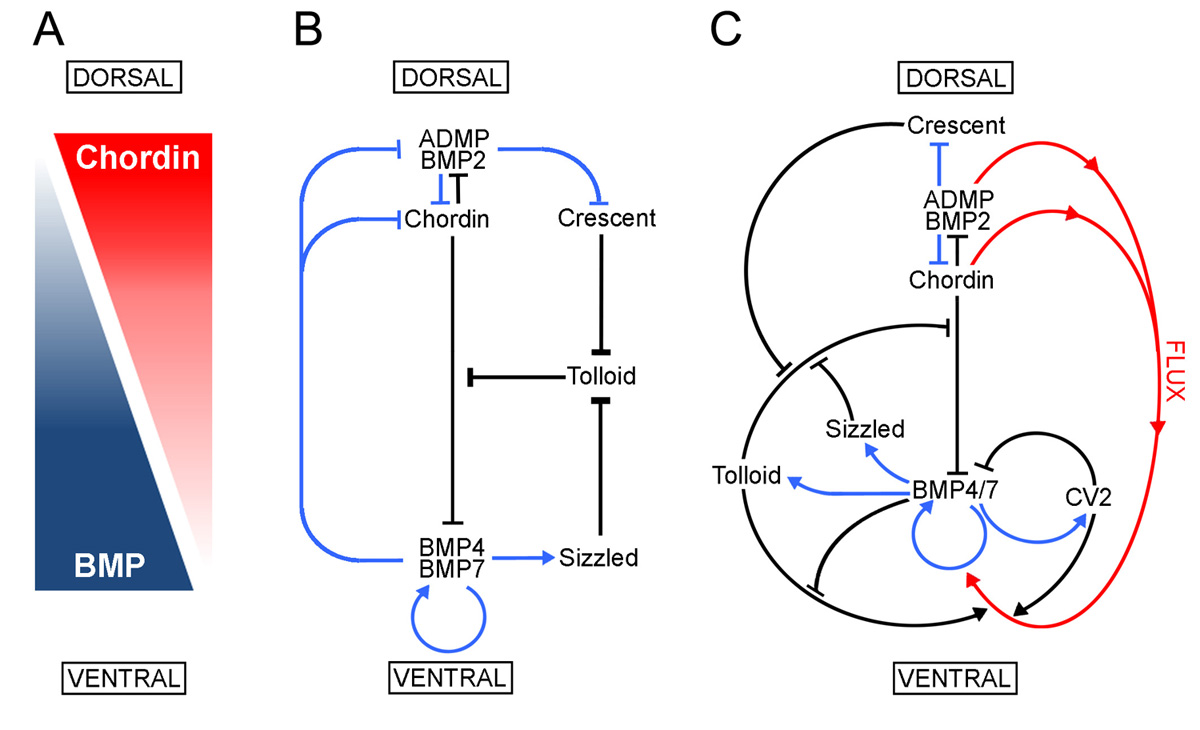 |
Model of the extracellular network of proteins that control D-V patterning. was shown here to be a component of the extracellular biochemical network that controls D-V patterning. Arrows in blue represent transcriptional regulation by BMPs, arrows in black symbolize direct protein-protein interactions, and the red arrows indicate flux of Chordin/ADMP/BMP from the dorsal toward the ventral side of the embryo. Each protein-protein interaction indicated here is supported by biochemical and embryological studies in Xenopus. |
A direct screen for secreted proteins in Xenopus embryos identifies distinct activities for the Wnt antagonists and Frzb-1
Edgar M. Pera and E. M. De Robertis
To determine the spectrum of secreted proteins that are present in the extracellular space of early Xenopus embryos, a direct secretion screen was performed. Surprisingly, 24% of previously identified bona fide secretory proteins corresponded to four secreted Wnt antagonists of the same family: frzb-1, sizzled, sfrp-2, and crescent. sfrp-2 and crescent are novel components of the growing cocktail of growth factor antagonists secreted by Spemann organizer cells in Xenopus. is first expressed at blastula, defining a deep endodermal region that may be homologous to the avian hypoblast. Unlike other members of this family of inhibitors, microinjection of crescent mRNA causes the development of cyclopic embryos, even though the amount of anterior neural tissue is normal. In crescent-injected embryos, studies with specific markers indicate that morphogenetic movements of the anterior midline are abnormal, resulting in a more posterior location of prechordal plate and ventral forebrain markers with respect to the developing eye field. The results are discussed in light of recent findings in zebrafish and Xenopus that suggest that Wnt signalling through non-canonical (non-catenin dependent) pathways plays a pivotal role in the regulation of morphogenetic movements.
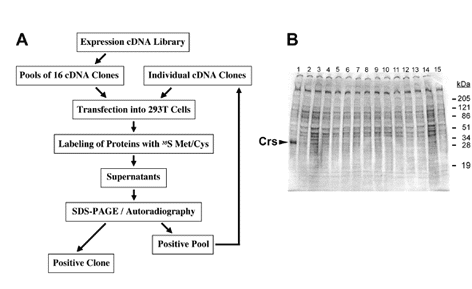 |
Fig. 1. Secretion cloning |
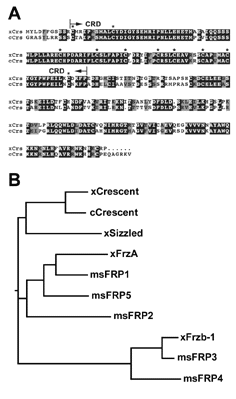 |
Fig. 2. Primary protein structure of |
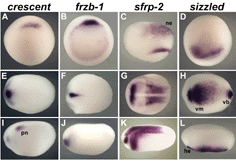 |
Fig. 3. Expression of crescent, frzb-1, sfrp-2 and sizzled in Xenopus embryos by whole-mount in situ hybridization |
 |
Fig. 4. Dynamic expression of crescent during early Xenopus development |
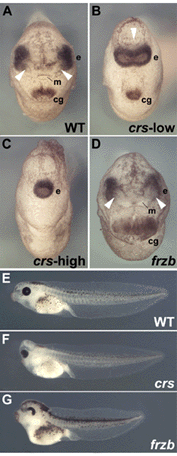 |
Fig. 5. Overexpression of crescent, but not of frzb-1, results in cyclopia |
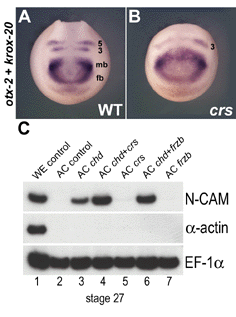 |
Fig. 6. Microinjection of crescent mRNA does not inhibit development of anterior neural structures |
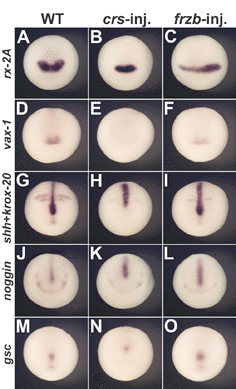 |
Fig. 7. Overexpression of crescent, but not of frzb-1 mRNA, perturbs the anterior extension of midline markers |
Crescent, a novel chick gene encoding a Frizzled-like cysteine-rich domain, is expressed in anterior regions during early embryogenesis
Peter Pfeffer, E. M. De Robertis and Izpisua-Belmonte, J.C.
Int. J. Dev. Biol. 41, 449-458 (1997)
We describe the isolation of a novel chicken gene that we have termed crescent, based on the most distinctive stage of its highly dynamic expression pattern during early embryogenesis. encodes a protein that in its N-terminal half shows the characteristic invariant 9 cysteine residues of the cysteine-rich domain (CRD) found in the Frizzled family of proteins, in Smoothened and in Collagen XVIII. The CRD of several Frizzled proteins have recently been shown to bind to Wg. Unlike Frizzled proteins, crescent does not contain a transmembrane domain and thus can not function as a receptor. expression is first found at stage XII (E-G&K) in the center of the area pellucida. On primitive streak formation, expression is detected in the entire anterior half of the area pellucida in the hypoblast layer. At maximal streak extension, crescent transcripts are localized primarily to the germinal crescent, where the primordial germ cells reside. During head process and head fold stages, crescent labels the anteriormost endodermal cells which will give rise to prospective foregut. With the commencement of somitogenesis, crescent expression rapidly wanes.